Additional information
| Raw Material | Moksifloksasin |
|---|---|
| Water Retention | None |
| Hepatotoxicity | Rare, mild liver enzyme elevations |
| Lab Test | Not specific |
| Also known as | BAY 12-8039 |
| Blood pressure | No significant effects |
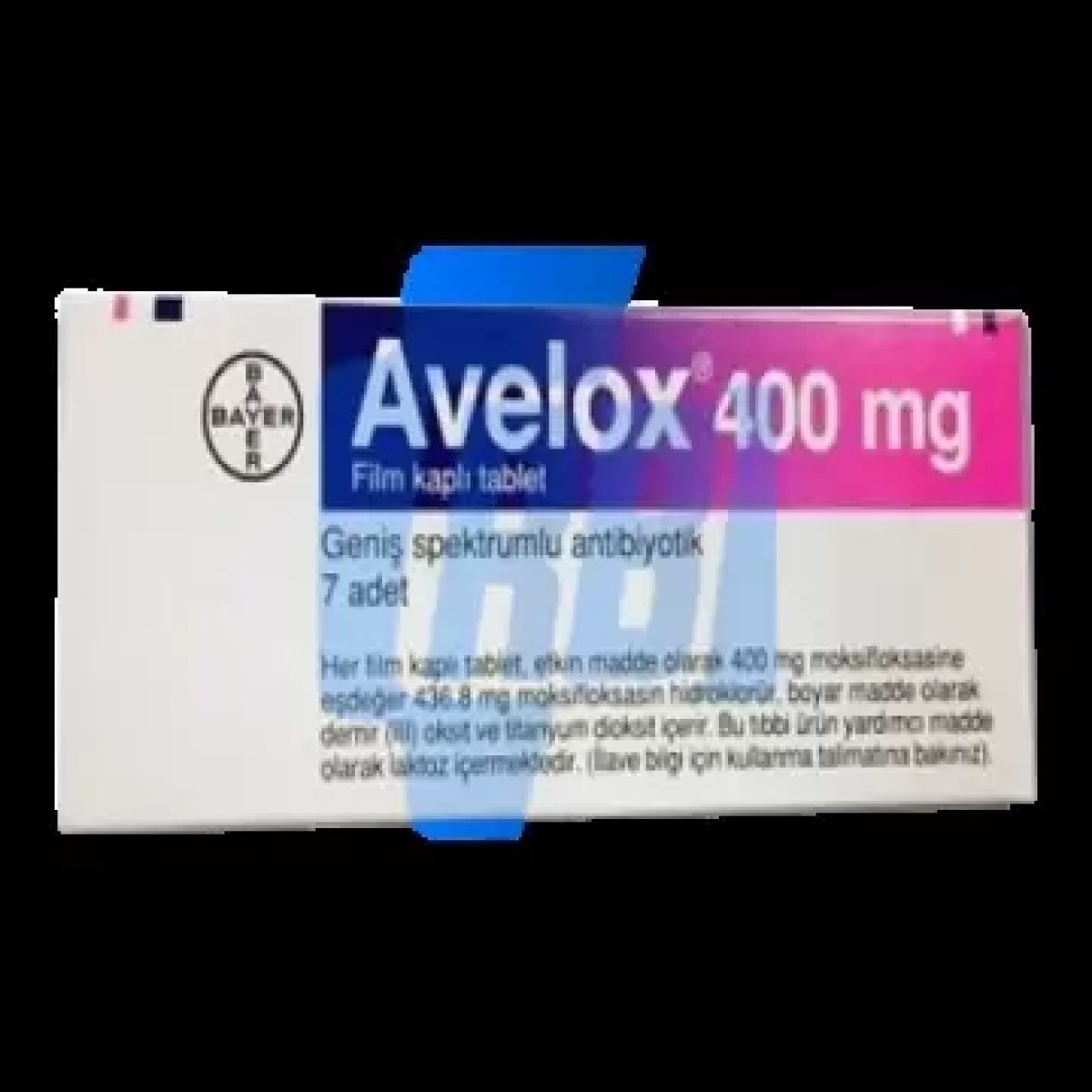
| Trade name | Avelox, Vigamox |
| Storage conditions | Store at room temperature away from moisture and heat |
| Chemical name | 1-Cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-4-oxo-3-quinolinecarboxylic acid |
| Formula | C21H24FN3O4 |
| Substance class | Fluoroquinolone antibiotic |
| Main action | Antibacterial |
| Half-life | Approximately 12 hours |
| Dosage (medical) | Typically 400 mg once daily |
| Dosage (sports) | Not applicable |
| Effects | Effective against a broad spectrum of gram-positive and gram-negative bacteria |
| Side effects | Nausea, diarrhea, dizziness, QT prolongation, tendon rupture |
| Use in sports | Not typically used in sports |
| Manufacturer | Bayer |
| Packing | 1 x 7 tabs (400 mg/tab) |






Reviews
There are no reviews yet.